Graduate School (UCSC) Research Summary
My graduate research was performed under Prof Pradip Mascharak in the Department
of Chemistry & Biochemistry. My two main projects were both inspired by the Mascharak
group’s interest in the bioinorganic chemistry of non-heme iron, specifically as
relevant to the enzyme Iron-Containing Nitrile Hydratase (Fe-NHase). This enzyme
exhibits a number of interesting features, as listed below:
* The iron center is found in a low spin, Fe(III) state and is not redox
active
* Fe(III) is ligated by two backbone carboxamido-N donors
*
Fe(III) is also ligated by two post-translationally oxygenated cysteinato-S donors
*
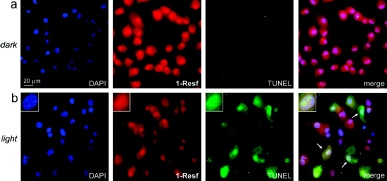
the inhibited “dark-form” Fe-NHase is bound by a nitric oxide (NO) ligand
*
the NO is photolabile, suggesting that the enzyme is light-regulated in nature
Project
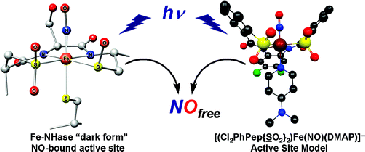
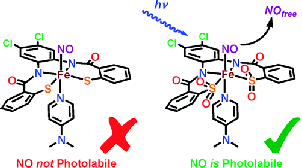
#1: Synthesis of a Biomimetic NO-bound Model of Iron-Containing Nitrile Hydratase
(Fe-NHase)
Previous work in the group established the structural, electronic and reactivity
properties of Fe(III) centers ligated to carboxamido-N, thiolato-S, sulfenato-SO
and sulfinato-SO2 moities. A number of previously studied ligands (PyPepSH4, PyPSH4,
PhPepSH4 among others) however did not allow synthetic access to any stable nitrosyl,
owing primarily to the high reactivity of the bound thiolato-S and its propensity
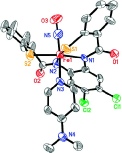
to promote high-spin Fe(III) rather than low-spin Fe(III). We prepared a new chlorinated
ligand Cl2PhPepSH4 that supported the octahedral geometry necessary to stabilize
a low-spin iron center.
Representative Publications
1) M. J. Rose, N. M. Betterley, P. K. Mascharak. Thiolate
S-Oxygenation Controls Nitric
Oxide (NO) Photolability of a Synthetic Iron Nitrile
Hydratase (Fe-NHase) Model Derived
from Mixed Carboxamide/Thiolate Ligand. J. Am.
Chem. Soc. 2009, 131, 8340-8341.
2) M. J. Rose, N. M. Betterley, A. Oliver, and P.
K. Mascharak. Binding and Photorelease
of Nitric Oxide (NO) to a Synthetic Model of
Iron-Containing Nitrile Hydratase (Fe-NHase).
Inorg. Chem. 2010, 49, 1854-1864.
Project
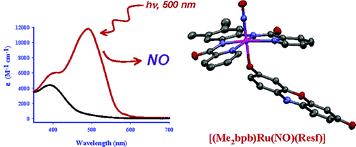
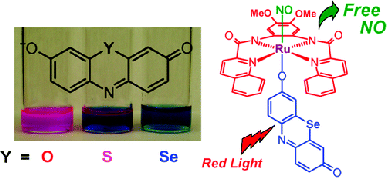
#2: Photosensitization of Ru Nitrosyls to Visible Light with Pendant Chromophores
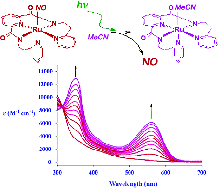
Although
ruthenium nitrosyls are more stable than their iron counterparts, the Ru-NO unit
is generally only photolabile to UV light, which is not compatible with biological
systems. For this reason, we prepared a number of complexes according to a strategy
of dye-sensitization, wherein a pendant chromophore is directly bonded to the metal
center. Phenoxazine chromophores (Y = O) sensitized the Ru-NO moiety to 500 nm light,
while its heavy-atom derivatives (Y = S, Se) sensitized to 600 nm light.
Representative Publications
1) M. J. Rose, M. M. Olmstead and P. K. Mascharak. Photosensitization
via Dye Coordination:
A New Strategy to Synthesize Metal Nitrosyls that Release NO
under Visible Light.
J. Am. Chem. Soc. 2007, 129, 5342-5343.
2) M. J. Rose, N. Fry,
R. Marlow, L. Hinck and P. K. Mascharak. Ruthenium Nitrosyls
bearing Coordinated Fluorophores
as NO Donors: a Novel Mode of Fluorometric Delivery
of NO to Cells with Visible Light.
J. Am. Chem. Soc. 2008, 130, 8834-8846.
3) M. J. Rose, P. K. Mascharak. Photosensitization
of Ruthenium Nitrosyls to Visible Light
with an Isoelectronic Series of Heavy-Atom
Chromophores: Experimental and DFT Studies
on the Effects of O-, S- and Se-Substituted
Coordinating Dyes. Inorg. Chem. 2009, 48, 6904-6917.
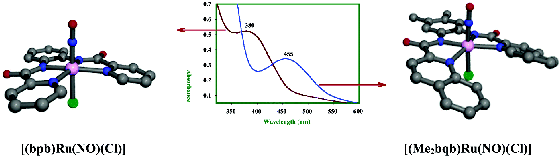
Project #3: Synthesis of Ruthenium
Nitrosyl Analogs for Biological NO Delivery
The photolabile properties of the low-spin
{Fe-NO}6 core found in Fe-NHase and biomimetics of the enzyme inspired the group
to investigate the possibility of deriving more stable nitrosyls derived from iron’s
2nd row cousin - ruthenium. Initial studies focused on N-donor ligands, due to their
air-insensitivity and general stability under biological conditions in experiments
requiring light-driven NO release (NO transfer to proteins, cells).
Representative Publications:
1) M. J. Rose, A. K. Patra, E. A. Alcid, M. M. Olmstead
and P. K. Mascharak. Carboxamido
and Schiff Base Ruthenium Nitrosyls: Isoelectronic
Complexes with Markedly Different Properties
of Photolability and Reactivity. Inorg.
Chem. 2007, 46, 2328-2338.
2) M. J. Rose, P. K. Mascharak. Photoactive Ruthenium Nitrosyls:
Effects of Light & Potential
as Biological NO Donors. Coord. Chem. Rev. 2008, 252,
2093-2114.