Research
Semiconductor/liquid interfaces play an
important role in the conversion of solar energy to fuel using
photoelectrochemical (PEC) cells. I look at the effect of
organic linkers on electron transfer across interfaces, the
effect of surface dipoles on band-edge positions, and the
tethering of fuel forming catalysts to semiconductor surfaces.
Hydride terminated Si surfaces are ideal for
PEC cells because they have very, very low trap state
densities, and the hydride is small, allowing for facile
charge transfer. That is, hydride terminated Si is ideal in
the absence of air and water. Upon exposure to air and water,
hydride terminated Si develops a thick, insulating Si oxide
layer that passivates the interface toward electron transfer -
in short, it shuts down the cell.
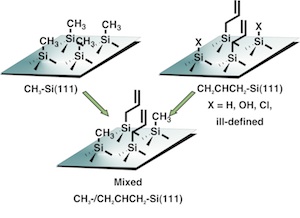
The Lewis group uses a two step
halogenation/alkylation reaction to stabilize Si surfaces in
the presence of air and water. The chemistry can produce a
Si(111) surface, on which every atop Si atom has a covalently
linked methyl group. These CH3-Si(111) surfaces are
stable for months in air. Additionally, photoanodes can be
functionalized in this way, and show stability in water
containing PEC cells.
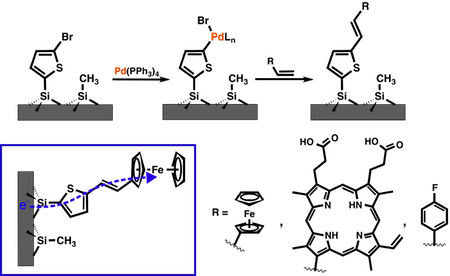
We have learned quite a lot about
stabilizing Si through research on CH3-Si(111),
however, there still issues that prevent the CH3-Si(111)
surface from becoming a fixed component in an efficient PEC
cell. One large issue being that the CH3-Si(111)
has poor kinetics for the HER reaction.